Cost vs Benefit: When Expensive Medications Make Sense Despite Side Effects
Drug Value Assessment Tool
Value Assessment Calculator
This tool helps you evaluate whether an expensive medication might be worth the cost based on the key factors discussed in the article.
Your Assessment Result
Note: This tool provides a general assessment based on the article's framework. Always consult with your healthcare provider and financial counselors for personalized advice.
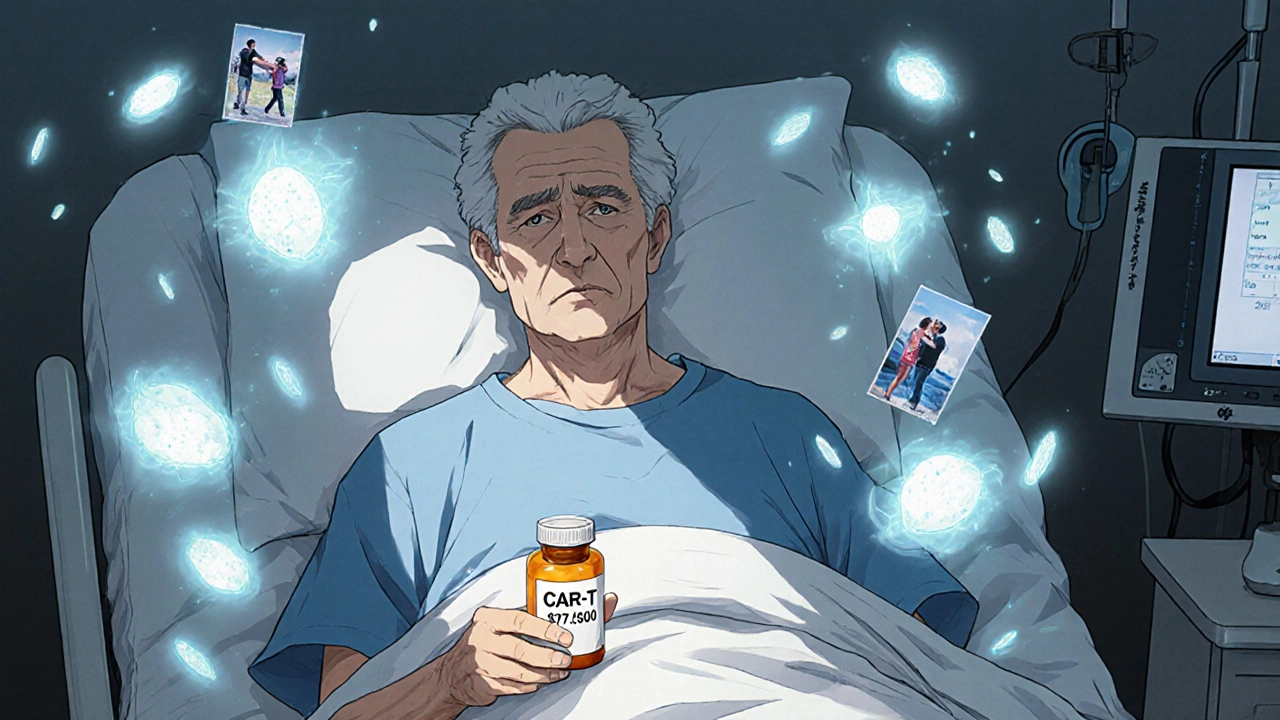
Find Financial AssistanceWhen a $500,000 treatment is the only real choice
Imagine your doctor says the only drug that might save your life costs half a million dollars. It comes with a 30% chance of severe fever, low blood pressure, and a hospital stay. Your insurance won’t cover it all. You’ll still owe $20,000. You’ve tried everything else. Nothing worked. What do you do?
This isn’t science fiction. It’s real life for people with certain cancers, rare genetic disorders, and autoimmune diseases. In 2024, the average cost of the 50 most expensive drugs in the U.S. was over $16,000 per dose. Some, like CAR-T cell therapies, cost nearly half a million dollars per treatment. And yet, for thousands of patients, these drugs are the only thing standing between them and death.
Why do we pay so much? And when does the price actually make sense-even when the side effects are brutal?
It’s not just the price tag-it’s what you’re trading for
Most people think expensive drugs are just overpriced. But that’s not the whole story. The real question isn’t how much it costs. It’s what you’re getting in return.
Take hepatitis C. Before 2014, treatment meant weekly injections of interferon for up to a year. Side effects? Severe fatigue, depression, flu-like symptoms, and a 50% cure rate. Today, a 12-week course of Harvoni cures 95% of patients with almost no side effects. The upfront cost? $7,153 out-of-pocket for many in 2016. But compared to the old treatment? It was a no-brainer. No more missed work. No more hospitalizations. No more lifelong liver damage.
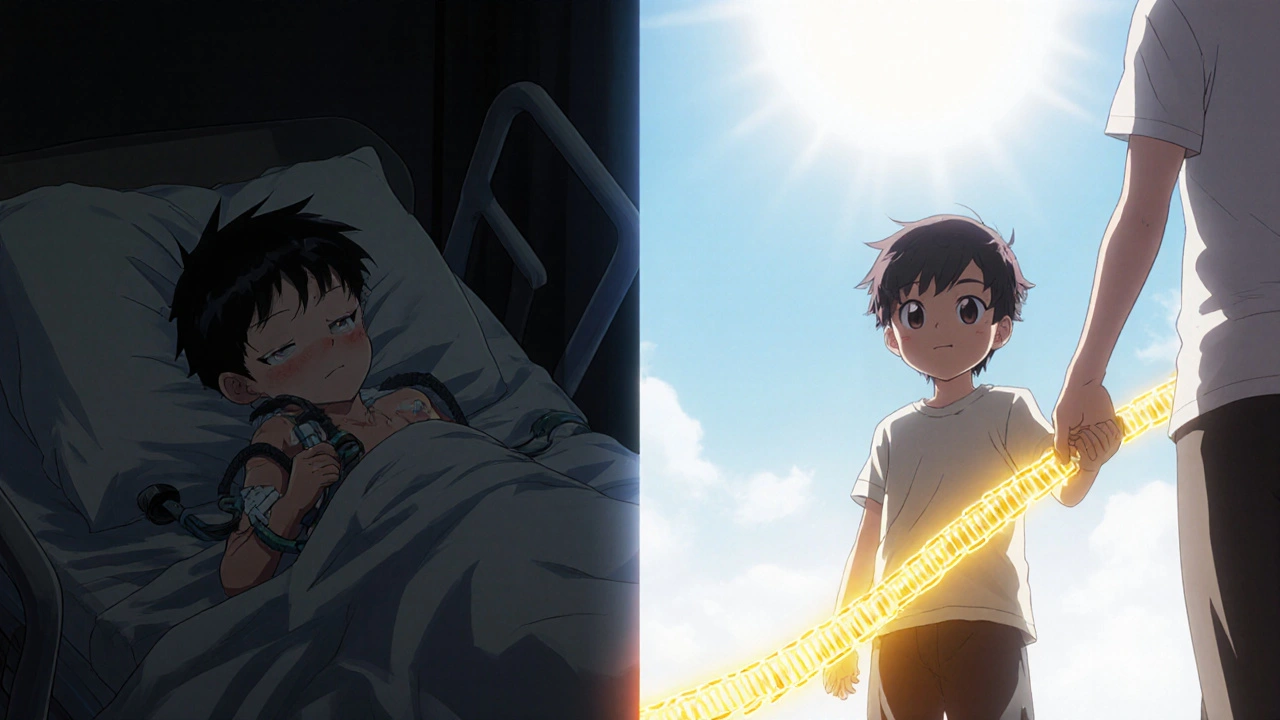
That’s the pattern. Expensive drugs often replace treatments that are cheaper but far worse. They don’t just extend life-they restore it. A patient with hemophilia who used to bleed into joints every month can now take a weekly pill and live without pain. A child with spinal muscular atrophy, once expected to die before age two, is now walking because of a $2.1 million gene therapy.
Side effects matter. But sometimes, the alternative is worse.
Why do these drugs cost so much? The real reasons
Pharma companies say they need high prices to recoup research costs. The Tufts Center estimated $2.6 billion to bring a new drug to market. But here’s the catch: that number includes failed drugs, marketing, and profit margins-not just R&D.
More importantly, many ultra-expensive drugs aren’t truly groundbreaking. A 2023 analysis by Prescrire International found only 7 out of 100 new drugs offered major progress over existing ones. Yet they still sold for six-figure prices.
So why do they cost so much? Three big reasons:
- Orphan drug incentives. The 1983 Orphan Drug Act gave companies 7 years of market exclusivity for drugs treating rare diseases (under 200,000 U.S. patients). That’s great for innovation-but it lets companies charge whatever they want. Of the 50 most expensive drugs in 2024, 72% were for rare conditions.
- No price controls in the U.S. Unlike Canada, Germany, or the UK, the U.S. doesn’t negotiate drug prices for most patients. Medicare can’t bargain until 2026, and even then, only 10 drugs are targeted. The rest? Manufacturers set the price.
- Rebates hide the real cost. The list price you see? It’s not what insurers pay. Drugmakers give huge rebates to pharmacy benefit managers, but patients still pay based on the inflated list price. That’s why someone with good insurance might pay $500 a month, while a Medicare beneficiary without subsidies pays $5,000 for the same drug.
The system isn’t broken because companies are greedy. It’s broken because there’s no real standard for what a drug is worth.
How doctors and insurers decide: The QALY test
Outside the U.S., countries like the UK and Germany use something called the quality-adjusted life year (QALY) to decide if a drug is worth buying.
One QALY = one year of perfect health. If a drug extends life by 2 years but with poor quality (pain, fatigue, frequent hospital visits), it might only count as 1.2 QALYs.
The UK’s NICE says a drug is worth it if it costs under £30,000 per QALY. If it costs £120,000 per QALY? It gets rejected-unless the manufacturer lowers the price. That’s how daratumumab, a multiple myeloma drug, went from denied to covered: the price dropped from £120,000 to £45,000 per QALY.
In the U.S., there’s no official limit. But hospitals and insurers use similar math behind the scenes. If a cancer drug costs $200,000 and adds 4 months of decent life? That’s $600,000 per QALY. Most payers won’t cover that unless the patient has no other options.
It’s cold math. But it’s the only tool we have to stop companies from charging $1 million for a drug that adds 3 weeks of life.
When side effects are worth it: Real patient stories
One Reddit user, a 42-year-old with rheumatoid arthritis, wrote: "I took Humira for 5 years. Got shingles twice. Lost 15 pounds from nausea. But I went from using a cane to hiking again. Worth it."
Another, a mother of a child with Duchenne muscular dystrophy: "The drug costs $300,000 a year. We have to apply for help every 6 months. We skip meals sometimes. But my son can still hug me. He’s not in a wheelchair yet. That’s more than we had two years ago."
And then there’s CAR-T therapy. A 68-year-old with advanced lymphoma, after three rounds of chemo failed, got tisagenlecleucel. The treatment cost $475,000. He spent 10 days in the hospital with high fever and confusion. But 18 months later? No cancer. No chemo. No more scans.
These aren’t outliers. In a 2023 ASCO patient survey, 78% of people who got CAR-T therapy said it was "worth the cost," even with the side effects. Why? Because they were out of options.
Side effects don’t make a drug bad. They make it human. And sometimes, human enough to live.
How to get these drugs without going broke
Knowing a drug is worth it doesn’t help if you can’t afford it. Here’s how real people get access:
- Manufacturer patient assistance programs. Most big drugmakers offer free or discounted drugs to low-income patients. On average, they cover 40% of out-of-pocket costs for commercially insured people.
- Nonprofit foundations. Groups like the Chronic Disease Fund and Patient Access Network Foundation gave out $2.1 billion in help in 2022. Apply early-it takes time.
- Medicare Part D has a catastrophic coverage phase. Once you hit $8,000 out-of-pocket in 2024, you pay only 5% for the rest of the year. Many patients don’t realize this kicks in after months of paying thousands.
- Specialty pharmacies. These aren’t your local CVS. They assign case managers who handle prior authorizations, appeals, and financial aid. They spend 3+ hours per patient. Use them.
- Ask for a price match. Some hospitals negotiate bulk discounts. If your neighbor got the same drug for $10,000 less, ask your doctor to call the manufacturer. It works more often than you think.
Don’t assume you can’t afford it. The system is stacked against you-but it’s not impossible.

The future: Will we ever fix this?
The Inflation Reduction Act of 2022 finally lets Medicare negotiate prices for 10 drugs starting in 2026. That’s a start. But 96% of the most expensive drugs are still off-limits because they’re too new, too rare, or too complex.
Europe is moving faster. By 2025, all new drugs there will be reviewed jointly by 10+ countries for both safety and value. The U.S. is still stuck in a patchwork of private insurers, hospital systems, and pharmacy benefit managers.
But change is coming. More insurers are using independent groups like ICER to evaluate drug value. In 2022, only 12% of new drugs were reviewed by these groups. By 2027, that could jump to 35%.
The goal? Stop paying $500,000 for a drug that adds 2 months of life. Start paying $100,000 for one that gives you 5 years.
Patients shouldn’t have to choose between their health and their home. But until the system catches up, that’s still the reality for too many.
When expensive drugs make sense: The checklist
Not every expensive drug is worth it. Here’s how to tell if yours is:
- Did it work when nothing else did? If you’ve tried 3+ alternatives and failed, this drug might be your only shot.
- Is the side effect profile better than the old treatment? Even if this drug causes nausea, if the old one caused liver failure? It’s a win.
- Does it improve daily life? Can you work? Sleep? Play with your kids? That’s more valuable than just living longer.
- Have you checked financial help? Don’t say no until you’ve talked to a case manager or applied for aid.
- Is there a cheaper alternative with similar results? Sometimes generics or biosimilars exist. Ask your pharmacist.
If the answer is yes to the first three, and you’ve explored help options? The cost might be worth it.
What’s next if you’re considering an expensive drug
Start here:
- Ask your doctor: "Is this the best option, or just the newest?"
- Call the drugmaker’s patient support line. They have counselors who help with paperwork.
- Search for your drug + "patient assistance program"-most have one.
- Ask your insurer for a prior authorization appeal form. Many approvals get denied the first time.
- Join a patient advocacy group for your condition. They know the tricks.
You don’t have to fight alone. The system is broken-but people are finding ways through it every day.

mike tallent
November 17, 2025 AT 09:04Joyce Genon
November 18, 2025 AT 20:55John Wayne
November 19, 2025 AT 10:11Robert Merril
November 19, 2025 AT 13:06Noel Molina Mattinez
November 20, 2025 AT 15:30Roberta Colombin
November 22, 2025 AT 13:37Julie Roe
November 23, 2025 AT 09:04jalyssa chea
November 24, 2025 AT 08:46Gary Lam
November 26, 2025 AT 05:53Peter Stephen .O
November 26, 2025 AT 18:09Andrew Cairney
November 27, 2025 AT 19:00Rob Goldstein
November 29, 2025 AT 01:26vinod mali
November 29, 2025 AT 06:14Jennie Zhu
November 29, 2025 AT 10:38