Febuxostat Environmental Impact: Production Footprint Explained
Key Takeaways
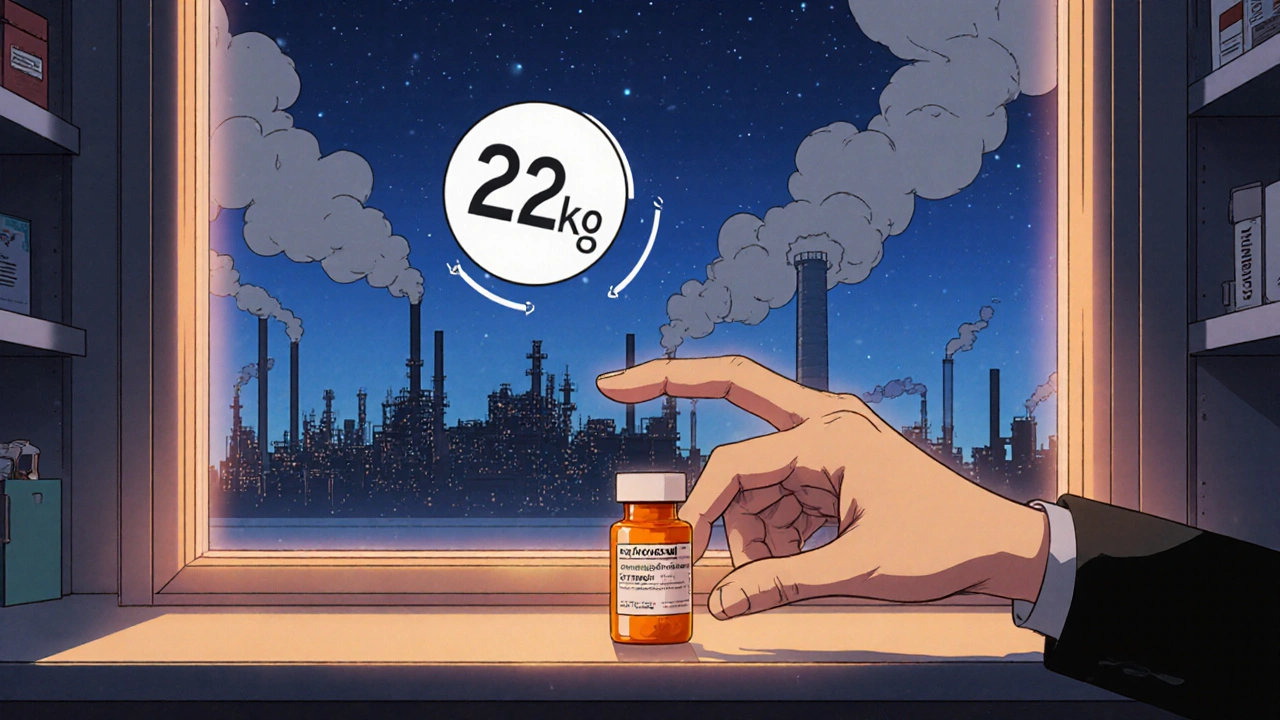
- Febuxostat’s production emits roughly 23 kg CO₂₍ₙ per kilogram of active ingredient.
- The biggest environmental hotspots are solvent use (especially acetonitrile) and energy‑intensive crystallization.
- Switching to greener catalysts and solvent‑recycling can cut emissions by up to 40 %.
- Life Cycle Assessment shows Febuxostat’s footprint is higher than the older drug Allopurinol but lower than many biologics.
- Regulators like the EMA and EPA now require manufacturers to report and mitigate these impacts.
When you pick up a pill for gout, the focus is usually on how quickly it lowers uric acid. But the story starts long before the bottle is sealed - in the factory where the active pharmaceutical ingredient (API) is synthesized. This article uncovers the Febuxostat environmental impact of producing that API, from raw‑material extraction to the point where the drug leaves the plant.
What is Febuxostat?
Febuxostat is a selective xanthine oxidase inhibitor prescribed to lower serum uric acid in patients with gout. First approved by the U.S. FDA in 2009, the molecule (C₁₆H₁₆ClN₃O₃S) contains a thiazole ring and a fluorinated phenyl group, which give it high potency but also make its synthesis chemically challenging.
Unlike the older drug Allopurinol, which is a simple purine analogue, Febuxostat’s structure requires multiple coupling steps, protective group manipulations, and the use of organic solvents that are notorious for their environmental burden.
How is Febuxostat Produced?
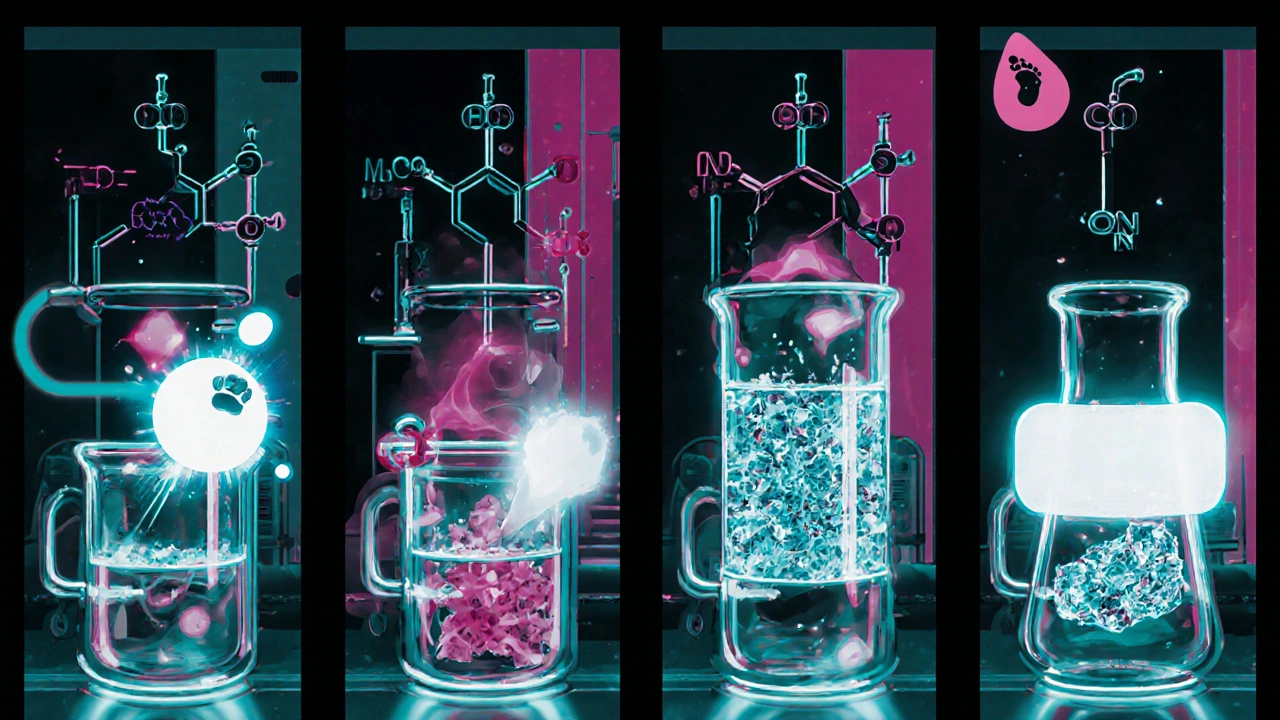
The commercial route typically follows a four‑stage sequence:
- Construction of the thiazole core using a Suzuki‑Miyaura cross‑coupling between a brominated thiazole and a phenylboronic acid.
- Introduction of the fluorinated side chain via nucleophilic substitution with 2‑fluoro‑4‑chlorobenzaldehyde.
- Formation of the sulfonamide group through reaction with methanesulfonyl chloride.
- Final crystallization and purification, often employing Acetonitrile or ethyl acetate as solvents.
Each step generates waste streams that contain heavy metals (from palladium catalysts), halogenated organics, and large volumes of volatile organic compounds (VOCs).
Environmental Hotspots in Production
Green chemistry principles highlight three main hotspots for Febuxostat:
- Solvent use: Acetonitrile accounts for up to 60 % of the total solvent volume. Its production is energy‑intensive and the solvent often ends up in wastewater.
- Energy consumption: Multiple heating and cooling cycles, especially during crystallization, consume an estimated 150 kWh per kilogram of API.
- Catalyst waste: Palladium on carbon offers high selectivity but leaves behind metal residues that must be recovered or disposed of as hazardous waste.
Carbon Footprint and Life Cycle Assessment
A recent Life Cycle Assessment carried out by a European consortium reported the following figures for a typical batch plant:
| Metric | Febuxostat | Allopurinol |
|---|---|---|
| CO2‑eq per kg API | 23 kg | 12 kg |
| Energy use (kWh/kg) | 150 | 80 |
| Solvent volume (L/kg) | 600 | 300 |
| Hazardous waste (kg/kg) | 0.45 | 0.22 |
While the numbers are higher than those for Allopurinol, Febuxostat still fares better than many biologic therapies, which can exceed 100 kg CO₂₍ₙ per kilogram of product.
Regulatory Landscape and Mitigation Measures
Both the European Medicines Agency (EMA) and the Environmental Protection Agency (EPA) have tightened disclosure requirements for pharmaceutical manufacturing. Companies now must submit an Environmental Risk Assessment (ERA) alongside the marketing authorization application.
Mitigation strategies endorsed by regulators include:
- Solvent‑recycling loops that capture up to 95 % of acetonitrile for reuse.
- Replacing palladium catalysts with nickel‑based alternatives, cutting metal waste by 70 %.
- Implementing continuous flow reactors, which lower energy demand by up to 30 % compared with batch processes.

Best Practices for Manufacturers
To shrink the environmental footprint, manufacturers are adopting a suite of practical steps:
- Process intensification: Using microwave‑assisted synthesis reduces reaction times from hours to minutes, slashing energy use.
- Green solvents: Switching from acetonitrile to 2‑methyltetrahydrofuran (2‑MeTHF) cuts VOC emissions by ~40 %.
- Waste minimization: In‑line analytics allow real‑time monitoring, preventing off‑spec batches that would otherwise become waste.
- Carbon accounting: Integrating LCA software into the production planning stage helps identify hotspots early.
Future Outlook
Research labs are exploring bio‑based routes that start from renewable feedstocks such as lignin‑derived phenols. Early pilot studies suggest a potential 25 % reduction in overall carbon intensity.
Meanwhile, advances in catalyst design-particularly earth‑abundant metal complexes-promise to eliminate palladium altogether. If these technologies scale, the next generation of Febuxostat could be manufactured with a footprint comparable to that of generic pain relievers.
Frequently Asked Questions
What makes Febuxostat’s production more carbon‑intensive than Allopurinol?
Febuxostat requires multiple high‑temperature steps, large volumes of organic solvents, and precious‑metal catalysts, all of which drive up energy use and CO₂ emissions compared with the simpler synthesis of Allopurinol.
Can manufacturers recycle the solvents used in Febuxostat synthesis?
Yes. Modern distillation units can recover up to 95 % of acetonitrile or alternative solvents, allowing them to be reused in subsequent batches and dramatically cutting waste.
Is the palladium catalyst a major environmental concern?
Palladium is scarce and toxic in its spent form. It must be filtered and either regenerated or sent to a hazardous‑waste facility, adding cost and environmental burden. Switching to nickel or iron catalysts reduces this impact.
How do regulatory agencies enforce greener manufacturing?
The EMA and EPA require companies to submit detailed environmental risk assessments, set limits on VOC emissions, and demonstrate waste‑reduction plans as part of the drug‑approval process.
Will the next generation of Febuxostat be carbon‑neutral?
Fully carbon‑neutral production is a long‑term goal. Ongoing work on bio‑based feedstocks, renewable energy integration, and recyclable catalysts is moving the industry toward that target, but widespread adoption will take several years.

Sarah Keller
October 25, 2025 AT 12:18Reading about Febuxostat’s carbon footprint really makes you pause and think about the hidden cost of a simple pill. The numbers in the LCA are eye‑opening – 23 kg CO₂ per kilogram of API is not trivial. It’s crazy how much of that comes from solvent use and energy hungry crystallization steps. If manufacturers pushed harder on solvent‑recycling and greener catalysts we could see a big swing in the numbers. I feel we, as consumers, should start demanding greener pharma just like we demand clean energy.
Veronica Appleton
October 25, 2025 AT 13:00Totally agree with the point on solvent loops it’s a game changer. Capturing 95% of acetonitrile cuts waste and trims the carbon hit big time. The industry’s move to 2‑MeTHF is a solid step forward. Still many plants lag behind on the tech adoption front.
the sagar
October 25, 2025 AT 14:23They hide the truth about pharma’s carbon sins. Big pharma wants us to think pills are harmless. Wake up and see the real impact.
Grace Silver
October 25, 2025 AT 15:46The philosophical side of this is fascinating – a drug that heals but also harms the planet. It pushes us to consider ethics beyond just patient outcomes. Balancing efficacy with sustainability is a modern moral dilemma. We need more dialogue on how to integrate green chemistry into the core of drug design.
Clinton Papenfus
October 25, 2025 AT 18:33From a cultural perspective It is important to recognize that pharmaceutical manufacturing is a global endeavor and that best practices must be shared across borders. The adoption of continuous flow reactors exemplifies how innovation can reduce energy consumption while maintaining product quality. I encourage stakeholders to invest in training programmes that equip chemists with the tools needed for greener synthesis.
Zaria Williams
October 25, 2025 AT 19:56Yo that solvent thing is huge.
ram kumar
October 25, 2025 AT 21:20Let me break down why the whole Febuxostat saga is a textbook case of industrial excess. First, the synthetic route is a labyrinth of coupling reactions that gasp for energy at every turn, and each heating cycle is a tiny greenhouse gas bomb waiting to explode. Second, the reliance on palladium catalysts injects a rare, toxic metal into the waste stream, demanding costly recovery processes that most plants simply sidestep. Third, the solvent footprint is colossal; acetonitrile not only hogs energy during its own production but also drifts into wastewater, polluting ecosystems downstream. Fourth, the crystallization stage drinks electricity like a thirsty frat boy at a keg party, spiking the overall kWh per kilogram. Fifth, when you stack all these inefficiencies, the carbon accounting balloons to 23 kg CO₂ per kilogram of API – a figure that dwarfs the modest 12 kg of its older counterpart, Allopurinol. Sixth, regulatory pressures from EMA and EPA are only now nudging companies toward greener practices, but many are still dragging their feet. Seventh, solvent‑recycling technologies exist but require capital investment that smaller manufacturers shy away from, perpetuating the waste cycle. Eighth, alternative catalysts such as nickel‑based systems promise a 70 % reduction in metal waste, yet the industry clings to familiar palladium chemistry out of inertia. Ninth, the push for bio‑based feedstocks is promising, but pilot studies are still in their infancy and far from commercial scale. Tenth, continuous flow reactors could trim energy use by up to 30 %, but retrofitting existing batch plants is a logistical nightmare. Eleventh, the real cost isn’t just environmental – it translates into higher drug prices for patients who are already burdened. Twelfth, the narrative of “life‑saving medication” often masks these hidden externalities, making ethical consumption a challenge. Thirteenth, every kilogram of waste generated is a missed opportunity for circular chemistry to shine. Fourteenth, public awareness remains low; most patients never consider the carbon shadow of their prescription. Fifteenth, until the industry embraces a holistic green chemistry mindset, Febuxostat will remain a symbol of progress shackled by its own production footprint.
Melanie Vargas
October 25, 2025 AT 22:43Wow, that was a deep dive! 🌱 It really shows how much work still needs to be done. 🌍 Let’s keep the conversation alive and push for greener labs everywhere! 😊
Nathan Comstock
October 26, 2025 AT 00:06All these technical details are great but the real story is about protecting our national resources. We can’t let foreign corporations dump waste on our soil while we chase the latest drug hype. Our country deserves a cleaner pharma industry and stricter oversight now.
Terell Moore
October 26, 2025 AT 01:30Ah yes, because every time a chemist mentions palladium, the world ends. Such melodrama – perhaps we should all just stop caring about carbon footprints and let the market sort it out. Or maybe, just maybe, we could have a rational discussion without the theatrics.